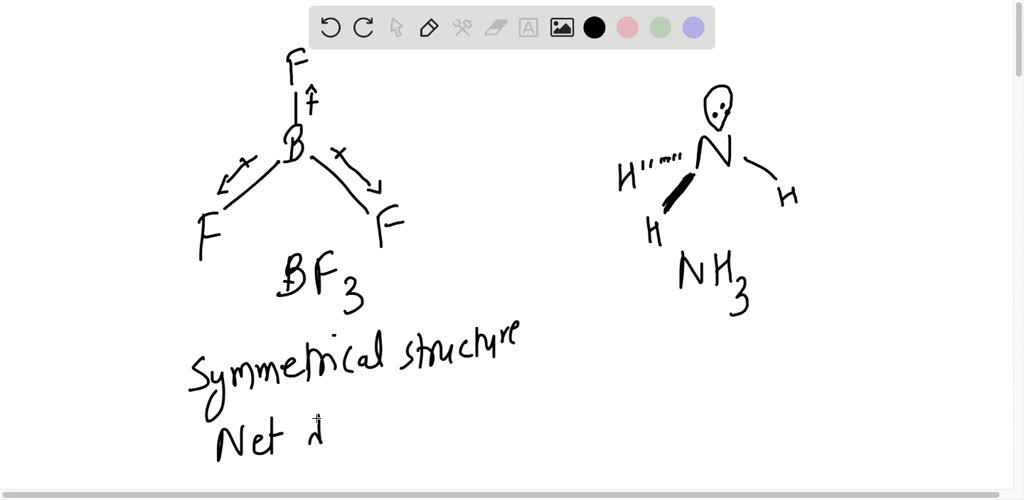
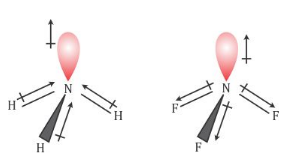
Can you explain why NH3 has such a large dipole moment compared with NF3? Show work. | Homework.Study.com
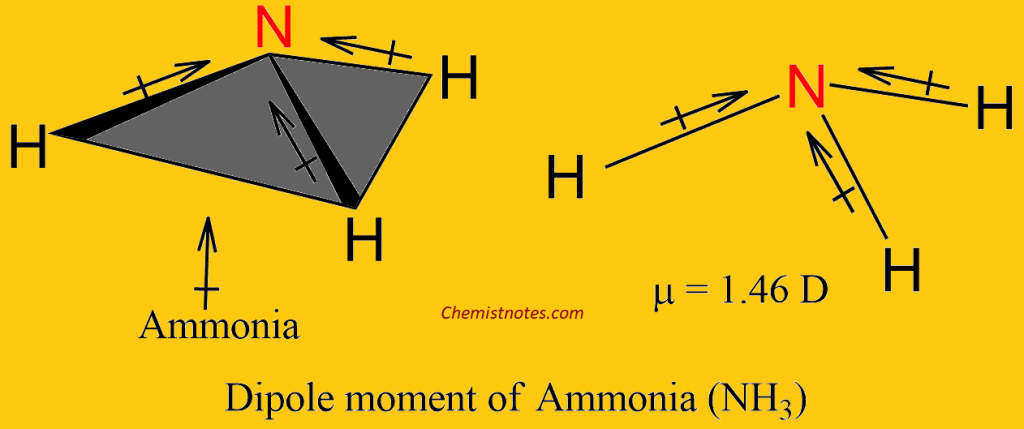
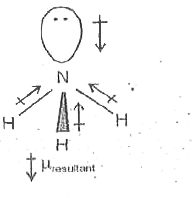
17. The electronegativity difference between N F is greater than that between N H. Yet the dipole moment of NH3 is 1.5D. This is because: 1. In NH3 the atomic dipole and

Is NH3 polar Or Nonpolar? - nh3 Intermolecular Forces, nh3 charge, nh3 bond angle, detailed facts? - chemwhite.com
Which out of ammonia (NH3) and NF3 has higher dipole moment and why? - Sarthaks eConnect | Largest Online Education Community

Effect of on dipole moments of CO, O3, NH3 molecules Dipole moment |P e... | Download Scientific Diagram
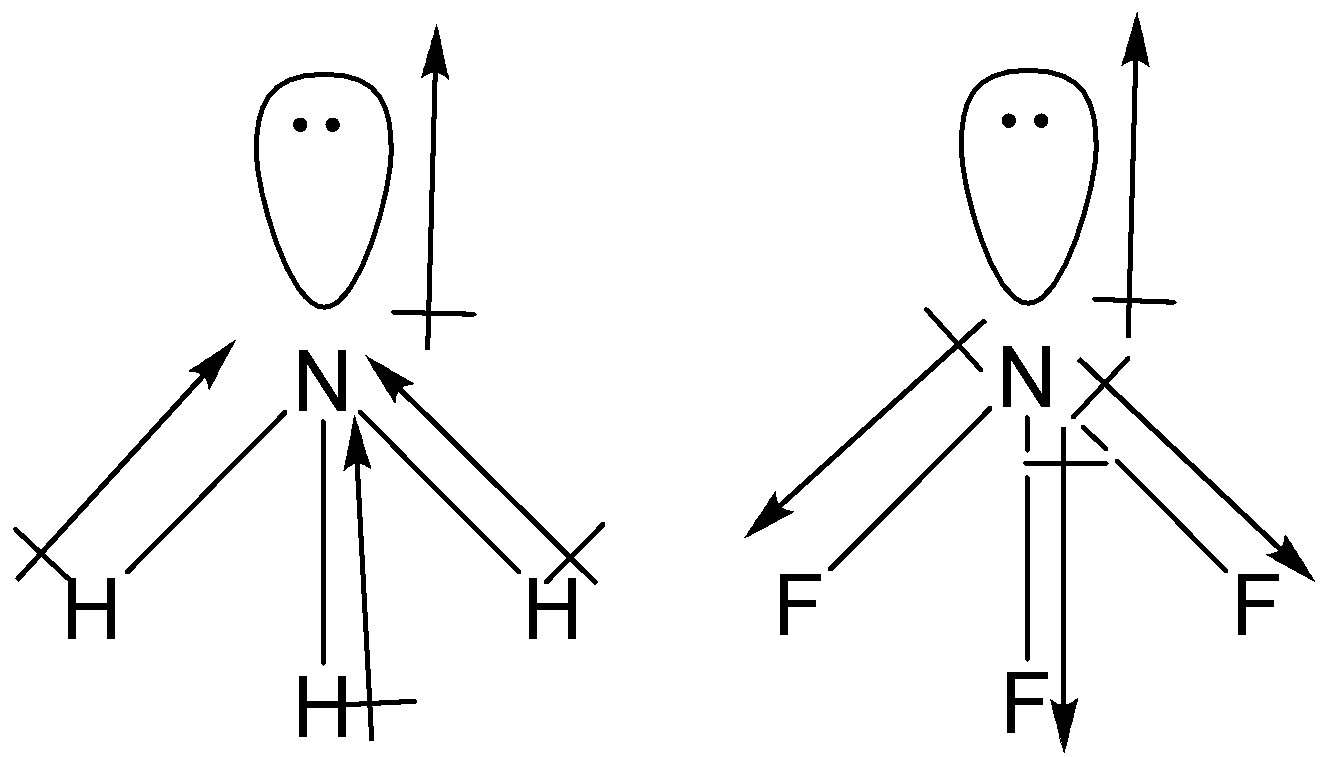

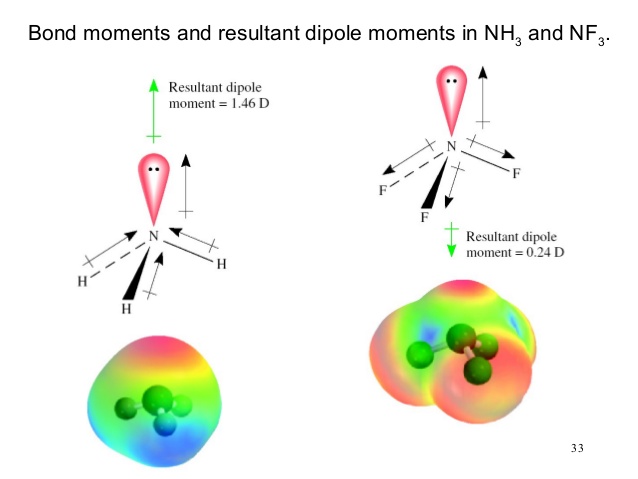








![Solved D) Has larger dipole moment (NH3, Or NF3] NH3 has | Chegg.com Solved D) Has larger dipole moment (NH3, Or NF3] NH3 has | Chegg.com](https://media.cheggcdn.com/study/a4f/a4f8fe7f-ba32-4d59-a27f-a1f5fd47c576/image.png)