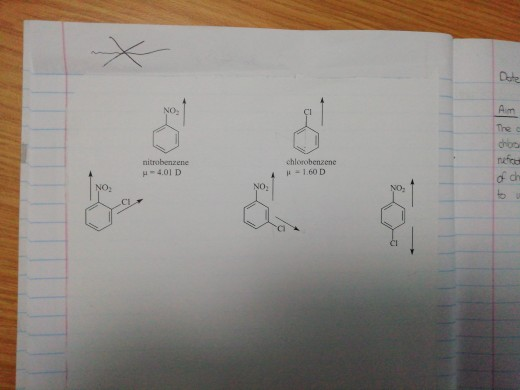
Arrange the following compounds in order of increasing dipole moments:I. ChlorobenzeneII. m dichlorobenzeneIII. o dichlorobenzeneIV. p dichlorobenzene

Determine if the given species has a permanent dipole moment. Chlorobenzene, C6H5Cl | Homework.Study.com

Explain why (i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? (i... - YouTube
The Dipole Moments of Chlorobenzene, Monochlorocyclopropane, and 1,2-Dichlorocyclopropane, with a Calculation of the Exterior Valence Angle of the Cyclopropane Ring1 | Journal of the American Chemical Society

Explain why the dipole moment of chlorobenzene is lower than cyclohexyl chloride ? – The Unconditional Guru
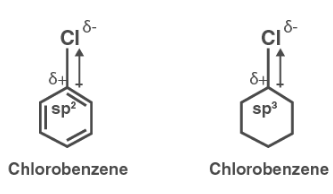
Explain why (i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? (ii) alkyl halides, though polar, are immiscible with water? (iii) Grignard reagents should be prepared under anhydrous

The dipole moment of chlorobenzene is 1.5D . Calculate dipole moment of 1,2,3,5 - tetrachlorobenzene.

Solve this: (d) Reactivity-Be Li K Cs The dipole moment of chlorobenzene 1 5 - Chemistry - The Solid State - 12295951 | Meritnation.com
e dipole moment of chlorobenzene is 1.5 D. Theighest dipole moment will be shown by(1) 1, 2 dichlorobenzene (2) 1, 2, 3 trichlorobenzene(3) 1, 2, 3, 4 tetrachlorobenzene(4) 1, 2, 3, 4, 5 pentacobenzene

Give reasons:(i) C - Cl bond length in chlorobenzene is shorter than C - Cl bond length in CH3 - Cl .(ii) The dipole moment of chlorobenzene is lower than that of
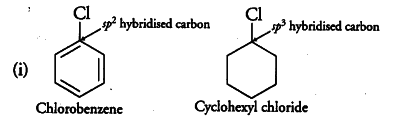
Explain why the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? - CBSE Class 12 Chemistry - Learn CBSE Forum
Explain why: (a) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? - Sarthaks eConnect | Largest Online Education Community

Pls Explain This Sum With Formula And How To Find Dipole Moment In Benzene Like Compounds Explain Me With Example - Chemistry - Stoichiometry - 10436977 | Meritnation.com

The experimentally observed dipole moments for chlorobenzene, toluene, and 4-chlorotoluene are given below. Based on these values, explain whether the methyl group acts as an electron withdrawing or electron releasing group. Comment
![The dipole moment of chlorobenzene is 1.73 D. The dipole moment of p di chlorobenzene is expected to be [CPMT 1991] (a) 3.46 D (c) 1.73 D (b) 0.00 ID (d) 1.00 D The dipole moment of chlorobenzene is 1.73 D. The dipole moment of p di chlorobenzene is expected to be [CPMT 1991] (a) 3.46 D (c) 1.73 D (b) 0.00 ID (d) 1.00 D](https://toppr-doubts-media.s3.amazonaws.com/images/1847473/f410b4fb-27a2-42b1-8a89-a5859a0c6cf0.jpg)






![Telugu] Explain why the dipole moment of chlorobenzene is lower than Telugu] Explain why the dipole moment of chlorobenzene is lower than](https://d10lpgp6xz60nq.cloudfront.net/physics_images/VIK_CHE_QB_C11_E02_016_S01.png)