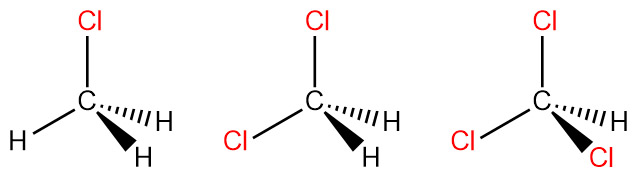
Draw a three-dimensional representation of the mentioned molecule and indicate the direction of any net dipole for the mentioned molecule. CH3Cl | Homework.Study.com

Is the molecule CH3Cl polar or nonpolar? If it is polar, specify the direction of its polarity. | Homework.Study.com
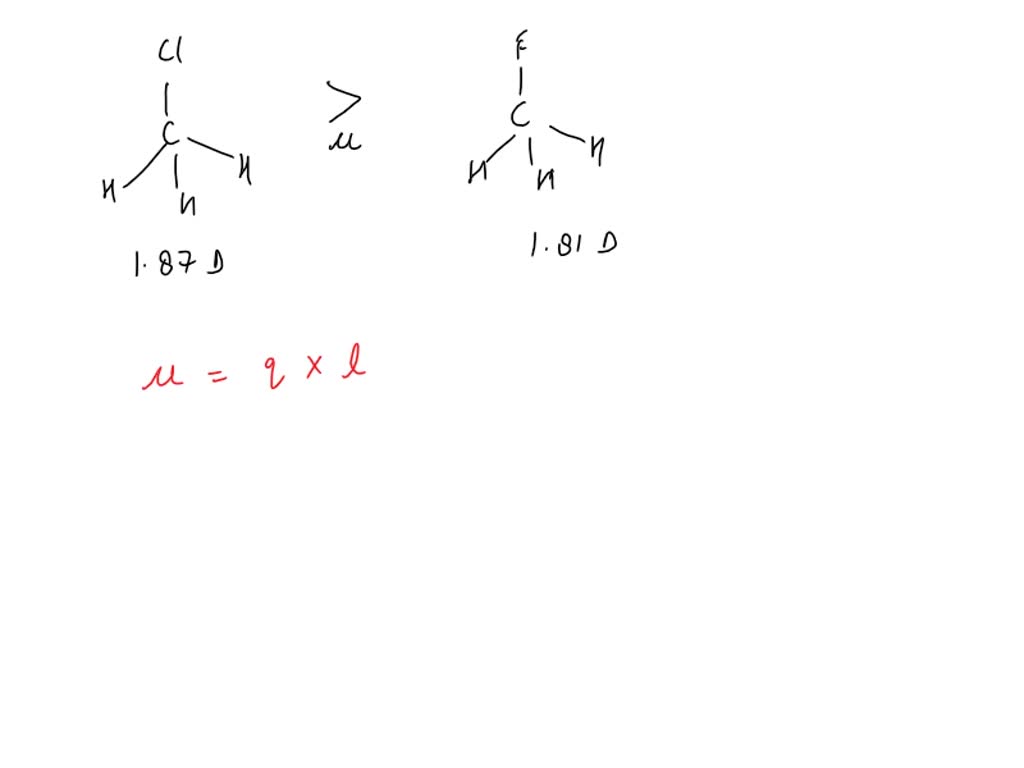
SOLVED: Fluoromethane (CH3F, μ=1.81 D) has a smaller dipole moment than chloromethane (CH3Cl, μ=1.87 D) even though fluorine is more electronegative than chlorine. Explain.
When we compare the dipole moment of CH3Cl, CH2Cl2 and CHCl3 we see that CH3Cl has the greatest value (which is greater than water too).Why does this happen? - Quora
![Best Answer] Which has highest dipole moment?? CH3Cl, CH2Cl2, CHCl3 or CCl4. well, I saw a video or Merit - Brainly.in Best Answer] Which has highest dipole moment?? CH3Cl, CH2Cl2, CHCl3 or CCl4. well, I saw a video or Merit - Brainly.in](https://hi-static.z-dn.net/files/df7/fd9f40d6eb64e74ec8cead9b4608b313.jpg)
Best Answer] Which has highest dipole moment?? CH3Cl, CH2Cl2, CHCl3 or CCl4. well, I saw a video or Merit - Brainly.in

Please explain the dipole moment of CH2Cl2,CH3Cl,CCl4,CHCl3 using diagram( direction )and arrange in increasing order of dipole moment - Chemistry - Haloalkanes and Haloarenes - 16270361 | Meritnation.com
Which of the following molecules has no dipole moment? (a) CH3Cl (b) CHCl3 (c) CH2Cl2 (d) CCl4 - Sarthaks eConnect | Largest Online Education Community
Welcome to Chem Zipper.com......: The Dipole moment of chloromethane is more than fluoromethane. Explain.
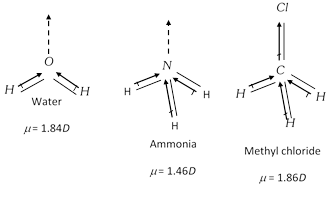
JEE Main, JEE Advanced, CBSE, NEET, IIT, free study packages, test papers, counselling, ask experts - Studyadda.com

halides - Why does chloromethane have a larger dipole moment than chloroform? - Chemistry Stack Exchange

Why does CH3Cl have more dipole moment than CH2Cl2 though by vector addition CH2CL2 should have more? - Quora