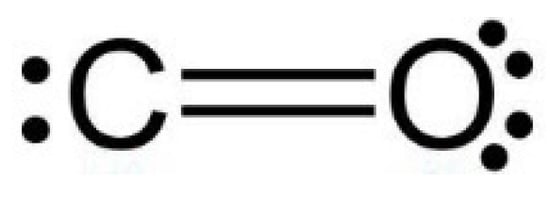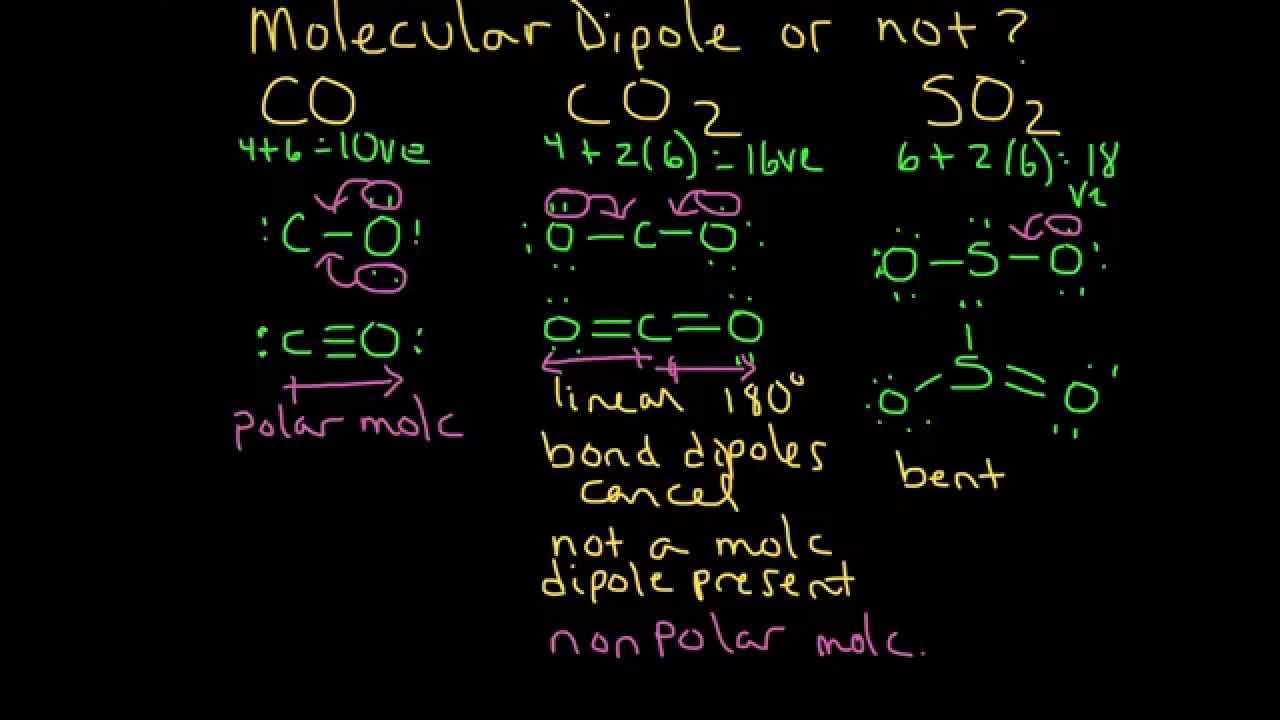Dipole moment functions of the CO molecule in the X 1 + and A 1 states.... | Download Scientific Diagram

Semi-empirical dipole moment of carbon monoxide and line lists for all its isotopologues revisited - ScienceDirect
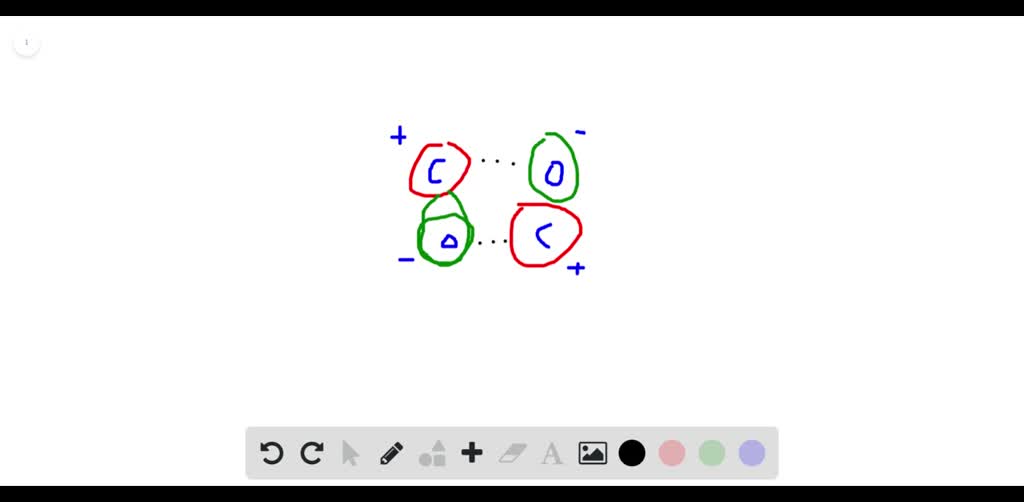
SOLVED:Draw the structure of the dipole-dipole interaction between two molecules of carbon monoxide.
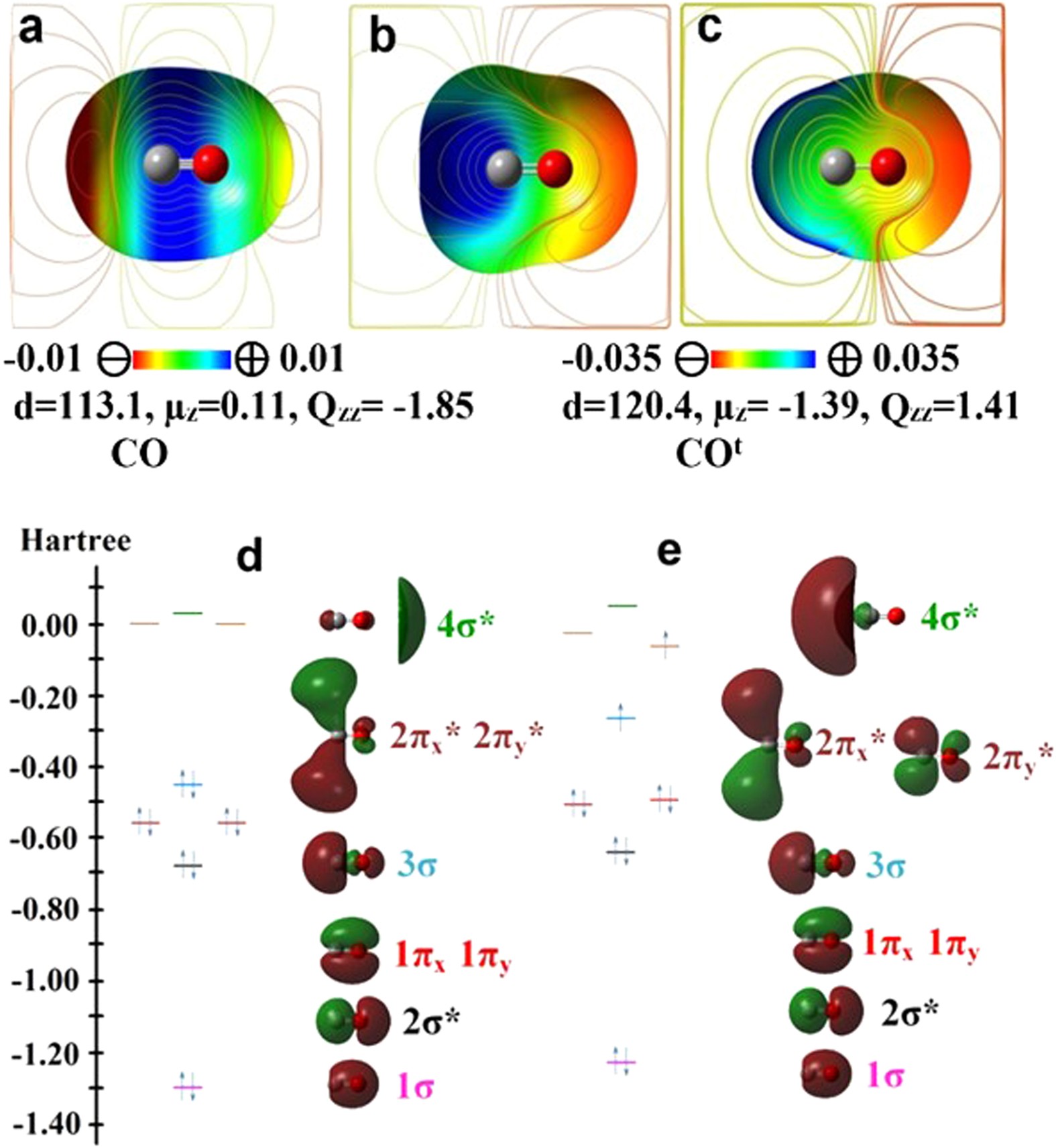
Intriguing Electrostatic Potential of CO: Negative Bond-ends and Positive Bond-cylindrical-surface | Scientific Reports

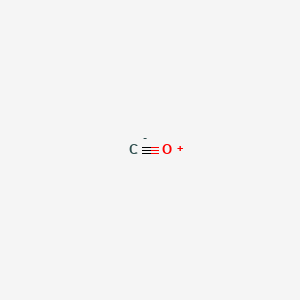
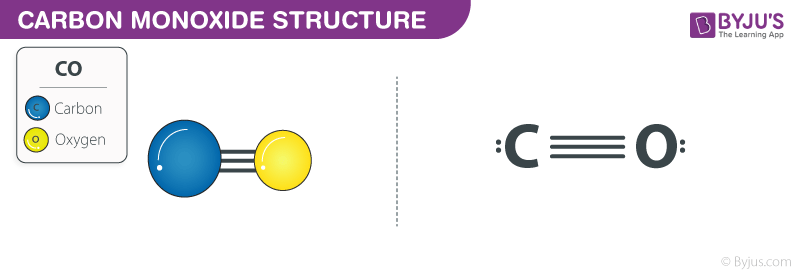
bond - How can the dipole moment of carbon monoxide be rationalised by molecular orbital theory? - Chemistry Stack Exchange

CHEMSOLVE.NET: Why CO is a poor Lewis base towards H + but it is an excellent Lewis base towards Ni and definition of synergic effect

Ground-state dipole moment of the spatially confined carbon monoxide and boron fluoride molecules - ScienceDirect
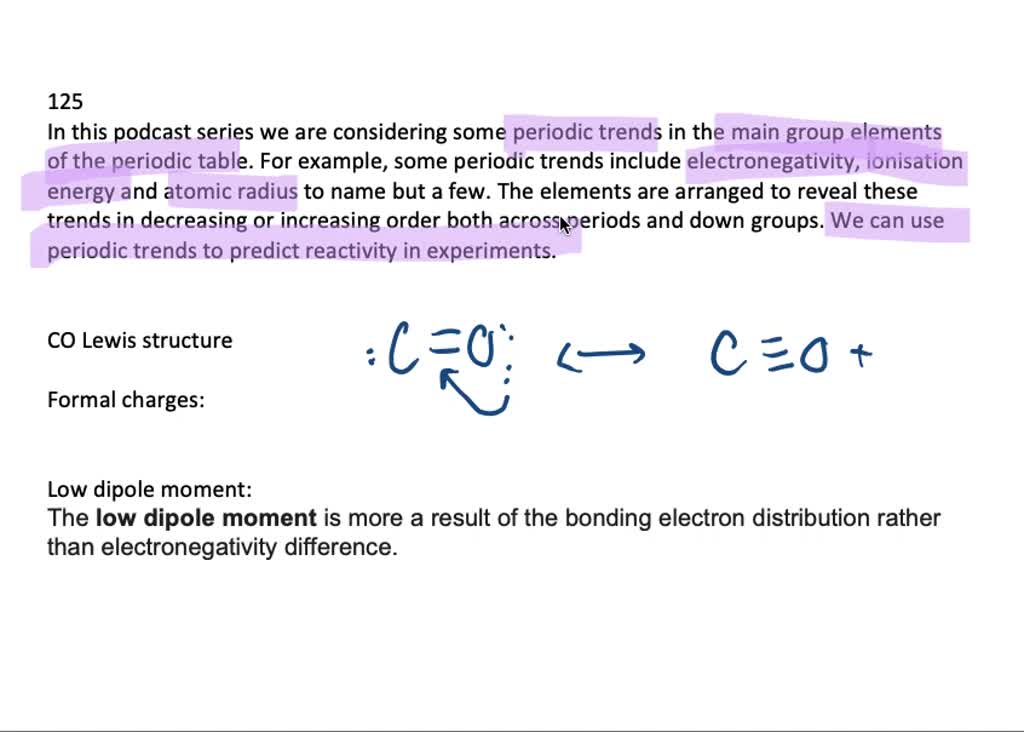
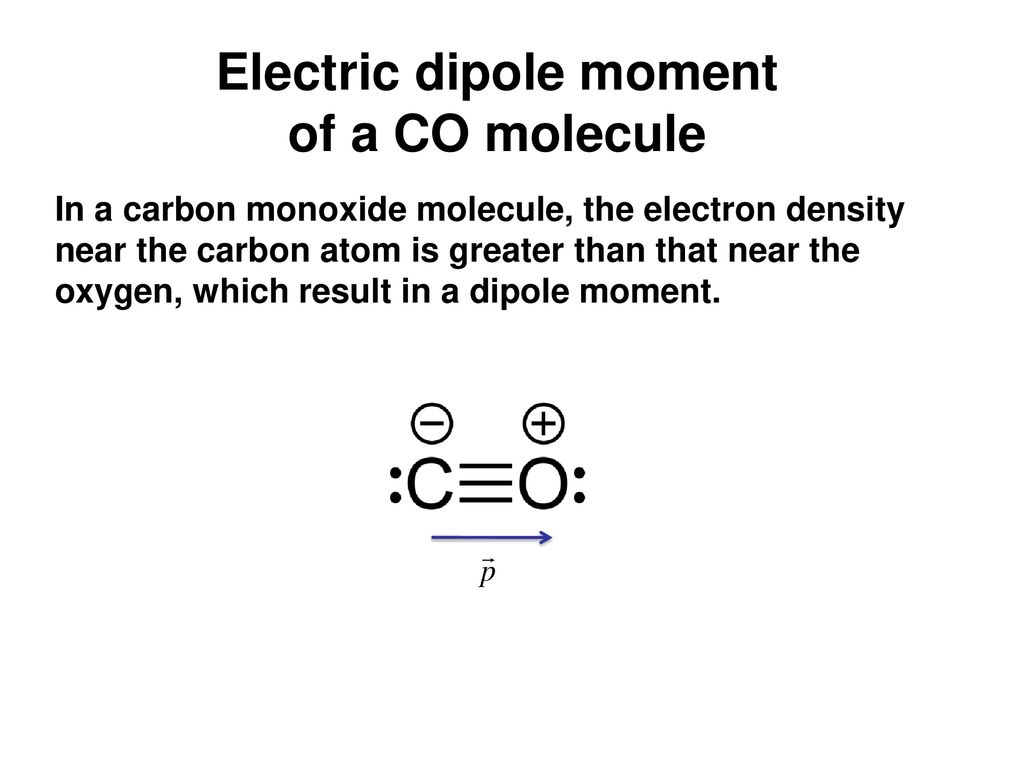
SOLVED:Look up the dipole moment for CO (carbon monoxide). How does it compare to the formal charges calculated from the Lewis dot structure? What does that tell you about the physical meaning

What are the most important types of interparticle forces present in the solids of carbon monoxide? | Homework.Study.com
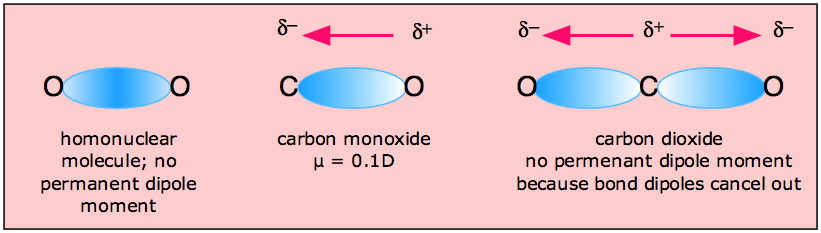
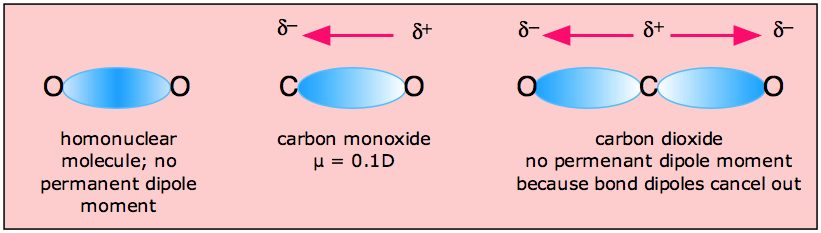
Why is the dipole moment of CO is very low? - Sarthaks eConnect | Largest Online Education Community
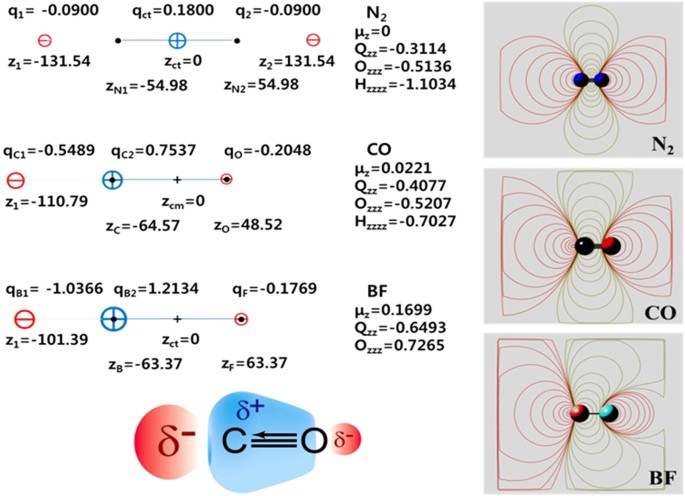
Intriguing Electrostatic Potential of CO: Negative Bond-ends and Positive Bond-cylindrical-surface | Scientific Reports