
The molecule with non-zero dipole moment?(a)- $BC{{l}_{3}}$ (b)- $BeC{{l}_{2}}$ (c)- $CC{{l}_{4}}$ (d)- $NC{{l}_{3}}$
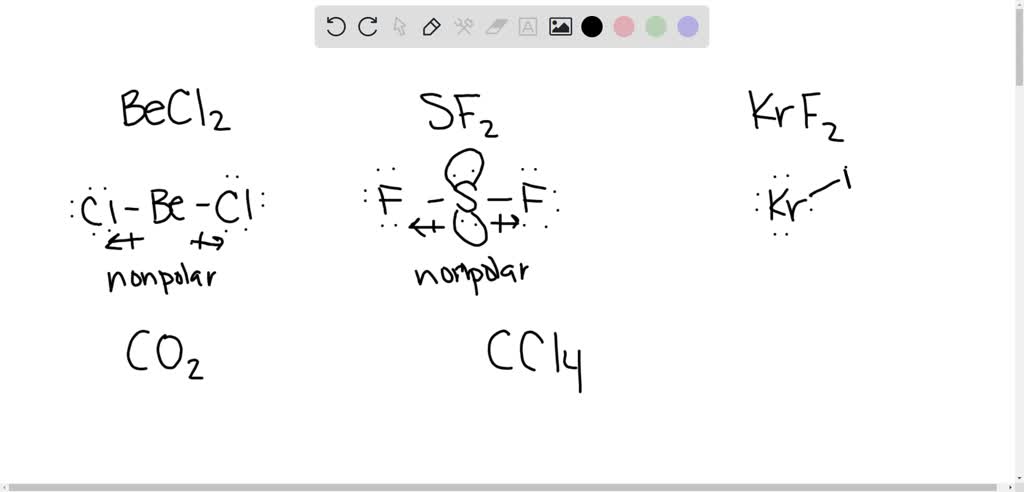
SOLVED: Which of the following molecules has a net dipole moment (which one is polar)? A) BeCl2 B) SF2 C) KrF2 D) CO2 E) CCl4
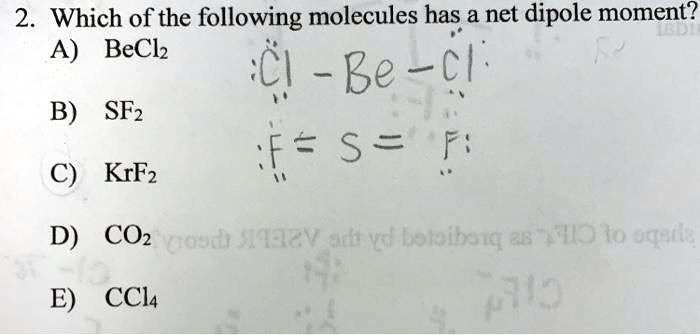
SOLVED: Which of the following molecules has a net dipole moment? A) BeCl2 (Be-Cl) B) SF2 (F-S-F) C) KrF2 D) CO2 E) CCl4

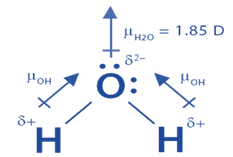
Must polar bonds give rise to polar molecules? And, why is water a polar molecule? | anhourofchemaday
Welcome to Chem Zipper.com......: What is hydrolysis product of BeCl2 with excess water at room temperature?

Question 17 Give reason the following: @ Dipole moment of BF3 is zero but ammonia has a dipole moment. (6) Cuci is covalent than Naci. C) LiCl is covalent than NaCl. (

Dipole moment of so2, h2o, ccl4, chcl3, cis & trans alkenes, co2, nh3, bf3, ch4 & organic compounds - YouTube

Predict whether the dipole moment of dibromomethane molecules is zero, close to zero, or significantly greater than zero. | Homework.Study.com
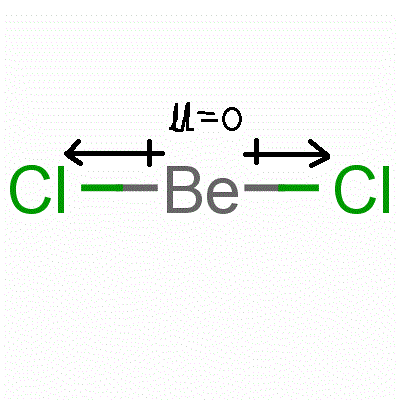

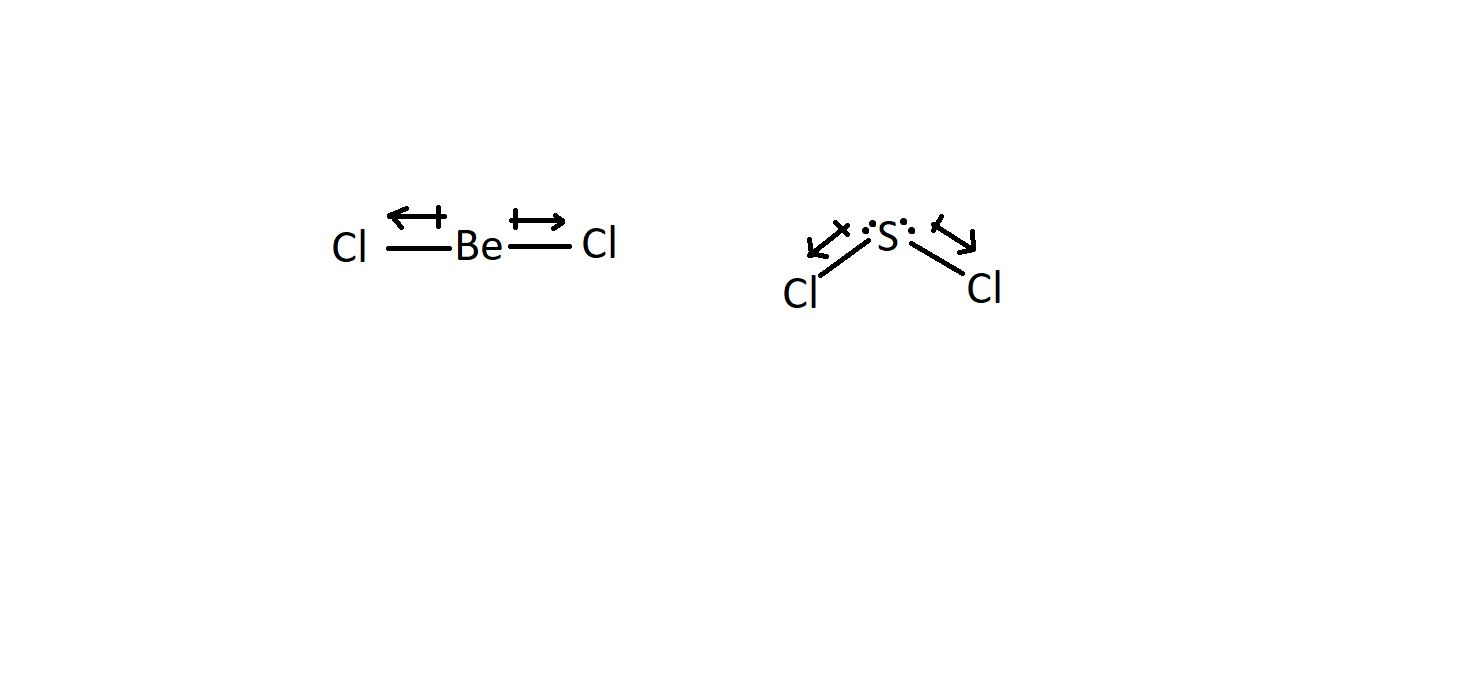







![Best Overview: Is BeCl2 Polar or Nonpolar [No#1] - Science Education and Tutorials Best Overview: Is BeCl2 Polar or Nonpolar [No#1] - Science Education and Tutorials](https://sciedutut.com/wp-content/uploads/2021/05/Is-BeCl2-Polar-or-Non-Polar-2-1024x493.png)

![Telugu] Assertion (A) : BeCl2, molecule is linear in shape.Reason (R) Telugu] Assertion (A) : BeCl2, molecule is linear in shape.Reason (R)](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/10812639.webp)


