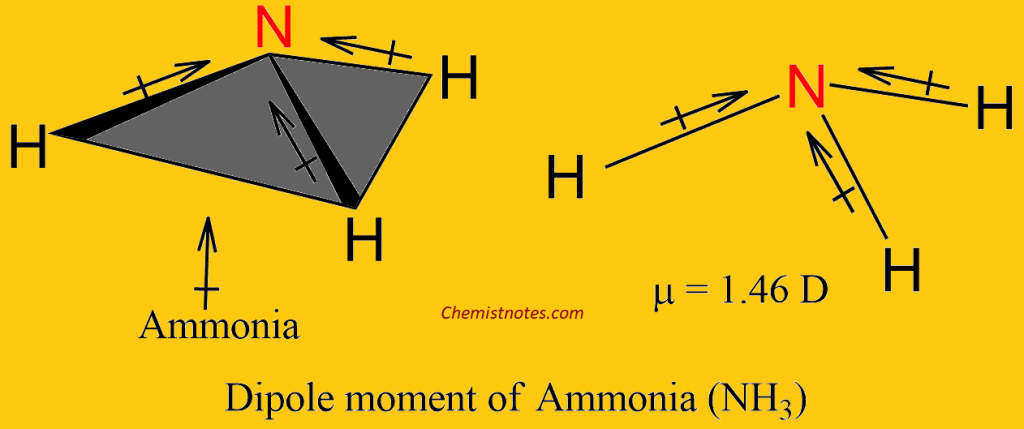
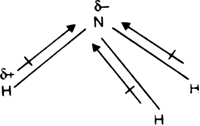
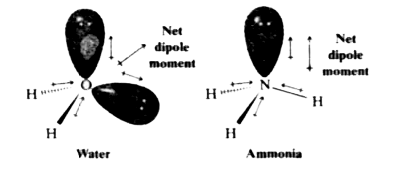
Dipole Moment as a Possible Diagnostic Descriptor of the Conformational Isomerism of the Ammonia Molecule | Semantic Scholar
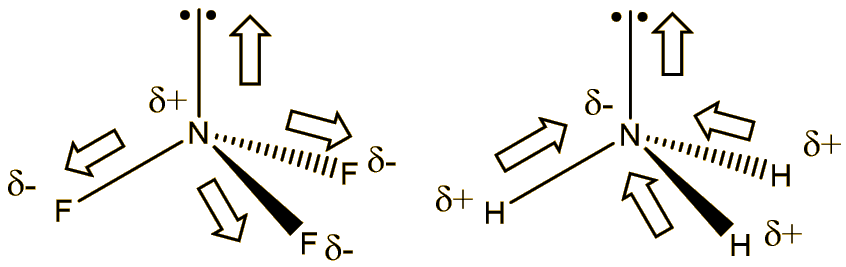
Why is the moment dipole for ammonia (1.64D substantially higher than that of ammonia trifluoride (0.24D) although the latter contains the highly electronegative flourine? - Quora

Sketch NH3 to show the correct molecular geometry and bond dipoles. Then state whether it is polar or nonpolar. | Homework.Study.com

Although flourine is more electronegative than nitrogen, the resultant dipole moment of ammonia is greater than nitrogen trifluoride Explain - Chemistry - Chemical Bonding and Molecular Structure - 6229927 | Meritnation.com
17. The electronegativity difference between N F is greater than that between N H. Yet the dipole moment of NH3 is 1.5D. This is because: 1. In NH3 the atomic dipole and
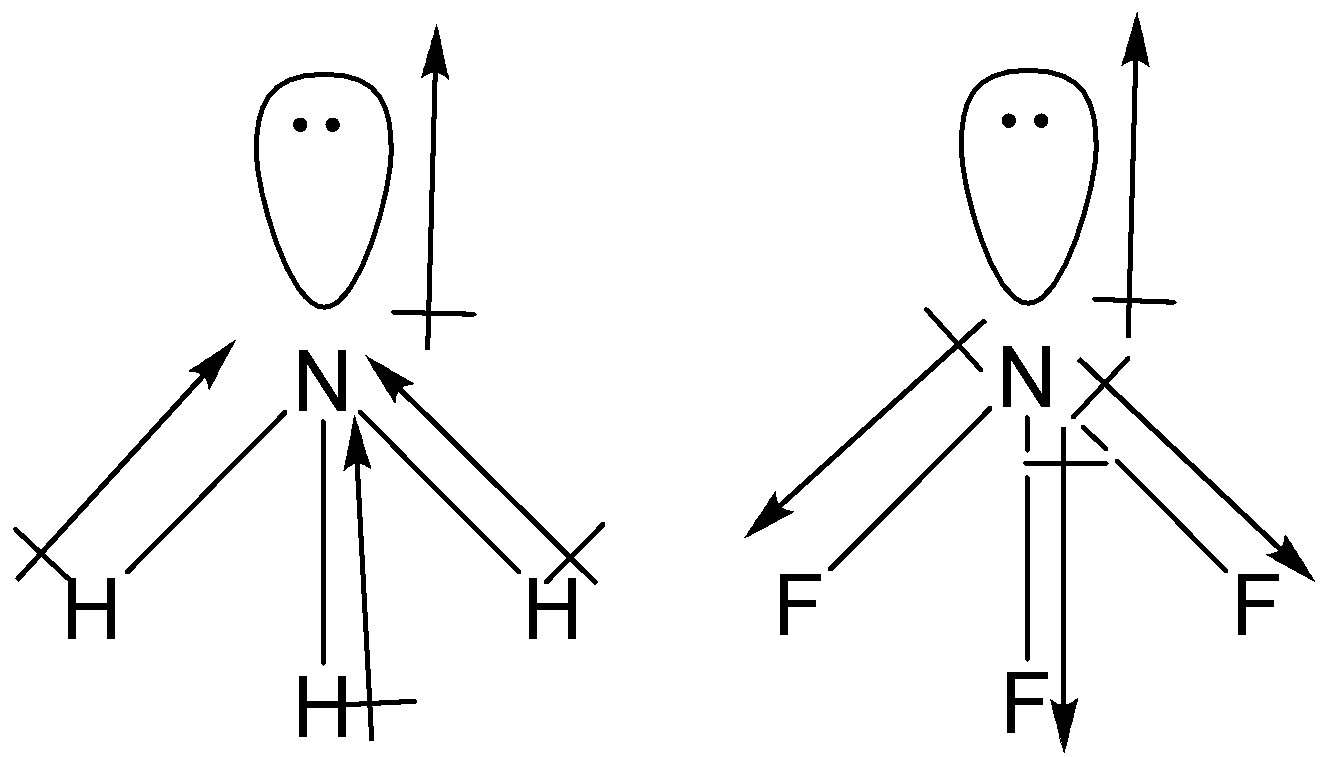
What is the correct dipole moment of $N{{H}_{3}}$ and $N{{F}_{3}}$ respectively?(A)- $4.90\\times {{10}^{-30}}$ C m and $0.80\\times {{10}^{-30}}$ C m(B)- $0.80\\times {{10}^{-30}}$ C m and $4.90\\times {{10}^{-30}}$ C m(C)- $4.90\\times {{10}^{-30}}$ C